Background: The prognosis for patients with multiple myeloma (MM) has improved over time, but there is considerable heterogeneity in disease outcomes. Currently used risk models have been validated using data from patients in clinical trials, but their applicability to real-world populations may be limited. To provide clinicians with better tools for decision-making, it is essential to identify factors associated with overall survival (OS) in a local setting. This study aims to identify clinical factors associated with OS in a real-world cohort of Colombian patients with newly diagnosed multiple myeloma (NDMM) using data from the registry on hematologic diseases (RENEHOC).
Methods: RENEHOC is an electronic healthcare record (EHR) system comprising data from 25 active centers in 6 Colombian cities as of July 2023. The study population was characterized using descriptive methodology. Two cohorts of patients were defined: Cohort A included patients who died within 5 years after diagnosis, and Cohort B included patients who were either alive or censored 5 years after diagnosis. Variables with a high proportion of missing information were evaluated in terms of magnitude and proportion. Univariate logistic regression was used to identify variables associated with OS, with those having a P value less than 0.2 considered for multivariate analysis. The results were presented as odds ratios (OR) with 95% confidence intervals (CI), and P values were calculated using the LR-test.
Results: The analysis included 1579 patients with NDMM. The median age was 67 years (IQR 60-75), and 51.1% (n=808) of the patients were male. Most patients were insured through the contributory health system plan. At diagnosis, 36.9% (n=582) of patients had a bone fracture (n=425) and 26.9% (n=425) had renal insufficiency, with 9.8% requiring dialysis. Cytogenetic analysis was performed in 22.9% (n=361) of patients, and 24.1% (n=87) of them had high-risk features. Half of the patients were classified as ISS III at diagnosis. The median OS for the entire cohort was 98.4 months. According to the international staging system (ISS), OS varied significantly: 106.2 months for ISS I, and 79.1 and 63.1 months for ISS II and ISS III, respectively (p < 0.001). First-line treatment for 90% (n=1407) of patients included chemotherapy, with 70% of patients treated with Bortezomib-based triplets, with VCD (Bortezomib, Cyclophosphamide, and Dexamethasone) being the most frequently used of them (30.7%, n=486). Only 21.7% of patients were consolidated with autologous stem cell transplantation (ASCT) in the first line.
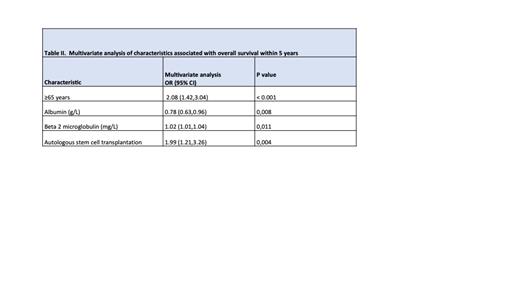
Cohort A comprised 341 (21.6%) patients who died within 5 years after diagnosis. Univariate analysis showed a significantly higher proportion of patients ≥65 years, from the subsidized health plan, with renal insufficiency requiring dialysis, elevated B2-microglobulin levels at diagnosis, and who did not receive ASCT in this cohort. After multivariate logistic regression, four variables remained statistically significant predictors of OS: age ≥65 years, elevated B2-microglobulin levels, not receiving ASCT, and higher albumin values at diagnosis. These variables were associated with higher mortality risk, except for higher albumin values, which were associated with better survival (Table I).
Conclusions: This study contributes to the growing body of evidence on multiple myeloma prognosis in the Colombian context. These findings provide valuable insights for clinicians in the local setting and may aid them in making informed decisions for individual patients. Nevertheless, future research and validation studies are warranted to confirm and build upon these findings.
Disclosures
Quintero:Merck Sharp and dome: Speakers Bureau; Takeda: Speakers Bureau; astra zeneca: Speakers Bureau; roche: Speakers Bureau. Galvez:Boehringer Ingelheim: Honoraria; AbbVie: Other: investigator on AbbVie-sponsored clinical trials; Sanofi: Research Funding. Quintero:Sanofi: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal